wanabe, I am a chemist of some kind, yes. I don't really want to get too specific on a public forum with strangers though, sorry. Inorganic molecules can sortof be represented by pictures like that; I mean, wikipedia has this picture of carbonate:
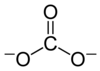
Whereas simplifying that would just take out the big "C." Most inorganics also form crystal structures as solids, and we use them in their solid form a lot, so it's usually helpful to have a more 3-dimensional picture. E.g. sodium carbonate has a crystal structure made of sheets like this:

Where you can see that every other carbonate has a sodium (purple sphere) above it--and the ones that don't have one below.
And obviously trying to capture all of this in a name is very difficult, but the crystal structure above is determined by the charge of carbonate (and the size of carbon/oxygen vs. sodium) so that is another reason to have the carbonate name so strongly emphasize charge.
Edit: Clarity.
















































